News
Catch up on the latest
Take a moment and read the latest stories about people who use our products and organizations that are doing good work. Feel free to share a particularly interesting tidbit with friends and family, too. And, if you have something to share, please send it to us.
Maverick junior and movido: the sturdy companions for children
Ottobock launches new prosthetic foot and hydraulic knee joints for children
March 10, 2025 - Austin, TX
- Providing the best possible fitting solution for children with disabilities is still not a matter of course.
- Ottobock is expanding its range of prostheses to enable children with amputations and dysmelia to enjoy an independent and empowered life.
- The Maverick junior prosthetic foot is the perfect companion for children: It is robust, waterproof and lightweight. Thanks to the flexibility of the fiberglass spring, children do not tire as quickly as with conventional prostheses, even after playing for a long time.
- With movido, the world’s smallest hydraulic knee joints, children learn to walk correctly with a prosthesis right from the start. Walking feels smooth and fluid, so they can have fun with friends without a care in the world.>
Whether it’s climbing a jungle gym, riding a bike, playing tag in the schoolyard, or kicking a ball around on the football field. Children want to play with their friends – whether they have two healthy legs or a prosthesis. Children’s prostheses need to be functional, durable and flexible, and should also prepare them for the use of adult fitting solutions.
This is why medical technology company Ottobock is expanding its product portfolio for children with the Maverick junior prosthetic foot and the movido prosthetic knee joints.
“It is important to us that every child with a prosthesis has the opportunity to lead an active and independent life. With the new solutions, we are taking the next step towards a more inclusive future,” says Maximilian Schillig, Global Product Manager at Ottobock. “With movido and Maverick junior, every movement – be it kneeling, running or playing – is possible with ease.”
Nora can go anywhere with the world’s smallest hydraulic knee joint
Nora lives near Chemnitz in Germany and was born with dysmelia in her left leg. This means that her leg is too short to walk. The world’s smallest and lightest hydraulic knee joint, movido, can help. The prosthesis from Ottobock combines all the functions that the 6-year-old needs to explore the world outside: it adapts to Nora’s walking speed, allows her to kneel easily, and is robust, compact, and especially lightweight at just 250 grams. movido is available in two sizes and will “grow” with Nora, adapting to her level of activity until she is ready to move on to an adult prosthesis.
The hydraulic swing phase control in the knee joint results in a more physiological gait pattern. In this way, Nora learns how to walk correctly with a prosthesis from the start. The 6-year-old can keep up with her friends and is fully prepared for all sorts of activities – be it playing on the jungle gym, riding a scooter or playing in the field with her sisters.
Toni overcomes every obstacle with his fiberglass prosthetic foot
Robust, waterproof and suitable for walking on a variety of surfaces – be it grass, sand or gravel. These features make the Maverick junior prosthetic foot the perfect companion for Toni’s everyday life. The 8-year-old from Bavaria in Germany is full of energy. He loves playing football and tag with his friends.
Maverick junior is made of fiberglass, making the prosthesis particularly lightweight and flexible. Combined with the low build height, the prosthesis is comfortable and has a smooth rollover when walking.
This means that Toni can play longer and doesn't get tired as quickly as he did with his previous prosthetic feet.
The movido prosthetic knee joints and the Maverick junior prosthetic foot for children are now available worldwide.
About Ottobock
For more than 100 years, Ottobock has been developing innovative fitting solutions for people with reduced mobility. As a Human Empowerment Company, Ottobock promotes freedom of movement, quality of life and independence. This is supported by more than 9,000 employees. Through their innovative strength, outstanding technical solutions and services in the fields of Prosthetics, Orthotics, NeuroMobility and Patient Care, they enable people in 135 countries to live the lives they want. Founded in 1919, the company continues to set new standards and drive the digitalization of the industry – together with its partners, medical supply companies and international research institutions – as the global market leader in wearable human bionics. Since 2018, Ottobock has been applying its expertise in biomechanics to exoskeletons for ergonomic workplaces. The company’s international activities are coordinated from its head office in Duderstadt in the German state of Lower Saxony. Ottobock has been supporting the Paralympic Games with its technical expertise since 1988.
Ottobock and Kennesaw State University Collaborate for Mobility Clinic, Empowering and Building Community Through Connection
October 4, 2024 - Austin, TX - Ottobock, a global leader in prosthetic and orthotic technology, has partnered with Kennesaw State University’s Wellstar College of Health and Human Services' Master of Science in Prosthetics and Orthotics (MSPO) program to host a transformative Full Circle Movement mobility clinic on October 12th, 2024. This event, taking place at Kennesaw State University’s Fifth Third Stadium, offers a unique opportunity for individuals of all ages and mobility levels to enhance their confidence, improve movement, and connect with a supportive community.
The clinic will focus on the power of movement, helping participants unlock their potential with their everyday prostheses while learning from Ottobock professionals and MSPO students and faculty. This collaboration underscores Ottobock’s commitment to innovation and community engagement, as well as Kennesaw State University’s dedication to educating future healthcare leaders in the field of prosthetics and orthotics.
“We’re thrilled to partner with Kennesaw State University’s MSPO program to bring this event to life,” said Scott Schneider, Head of Government/Medical Affairs & Future Development at Ottobock. “This clinic exemplifies our shared mission of improving lives through mobility, while offering students hands-on experience in working with patients to achieve their mobility goals.”
Event Highlights:
Date: October 12, 2024
Time: 10 a.m. - 1 p.m.
Location: Kennesaw State University, Fifth Third Stadium
3200 George Busbee Pkwy NW, Kennesaw, GA 30144
What to Expect:
- Enhance Confidence and Balance: Participants will receive personalized and group sessions to boost their confidence and improve balance while using their everyday prosthesis. Expert guidance from Ottobock professionals and MSPO students & faculty will ensure a safe, effective experience.
- Connect with a Supportive Community: Attendees will engage with local amputees, prosthetists, and healthcare professionals, sharing insights and stories to foster a sense of community and mutual support.
- Support for the "So Every Body Can Move" Initiative: The clinic will also spotlight Georgia’s ongoing legislative efforts through the So Every Body Can Move initiative, aimed at expanding access to mobility solutions and ensuring equitable opportunities for individuals with physical disabilities.
“The Full Circle Mobility Clinic reflects Wellstar College’s commitment to advancing health sciences through cutting-edge partnerships,” said Dr. Monica Swahn, Dean of Wellstar College. “Collaborating with Ottobock provides our students and faculty an unparalleled opportunity to engage in transformative care solutions, fostering innovation to benefit our community and set a new standard in mobility healthcare.”
For more information about the Full Circle Mobility Clinic and the collaboration between Ottobock and Kennesaw State University, follow our social channels: @ottobock_northamerica @wellstarcollege.
About Ottobock
Ottobock is a global leader in prosthetics, orthotics, and mobility solutions, dedicated to restoring freedom of movement for people with physical impairments. With over 100 years of innovation, Ottobock continues pioneering new technologies that improve quality of life.
About Kennesaw State University Wellstar College of Health and Human Services
The Wellstar College of Health and Human Services at Kennesaw State University provides comprehensive healthcare education, including its renowned Master of Science in Prosthetics and Orthotics program, preparing future healthcare professionals to make a lasting impact in their field.
Ottobock Leads the Charge in Expanding Access to Prosthetics for Underserved Patients
July 19, 2024 - Austin, TX - Medicare officially announces LCD changes that expand access to microprocessor-controlled knees (MPKs) for lower mobility users—a group that is often overlooked as users of this groundbreaking technology (view the changes here). Ottobock has been the driving force behind the expansion of Medicare coverage of MPKs for low-mobility patients, leading the way with two decades of research and advocacy. As the world’s largest prosthetics and orthotics manufacturer, Ottobock is proud to continue its unwavering commitment to improving access to advanced prosthetic technology for underserved patient communities.
In 2005, Ottobock initiated the first clinical study involving patients in a low-mobility user group utilizing MPKs, leading to two pivotal publications. Despite the early evidence, it wasn't until 2014 when Dr. Andreas Kannenberg published the first systematic review of five MPK studies involving patients with limited mobility. However, this was still insufficient to sway Medicare at that time. Undeterred, Ottobock continued to fund more than 80% of subsequent studies, reinforcing its dedication to this critical research.
By 2021, the cumulative efforts paid off when Dr. Kannenberg and Dr. Andreas Hahn, VP of Clinical Research & Services at Ottobock published another comprehensive systematic review, encompassing 704 patients across 15 publications and 13 clinical trials. This rigorous scientific groundwork culminated in Ottobock filing the LCD Reconsideration Request in March 2022. This request has led to the current revision of the LCD for Lower Limb Prostheses, which, upon this finalization, expand Medicare coverage of MPKs to a whole new demographic of patients who meet specified conditions.
"The journey to expanding Medicare coverage for microprocessor knees to this group of patients has been long and challenging, but immensely rewarding. Our extensive research and clinical trials have consistently shown the profound impact that advanced prosthetic technology can have on individuals with limited mobility,” stated Dr. Andreas Kannenberg, the Executive Medical Director of Ottobock North America. “This expansion is not just a testament to the hard work and dedication of our team at Ottobock; it also underscores the importance of innovation and evidence-based practice in transforming healthcare and enhancing patient outcomes. We look forward to continuing our efforts to ensure that every patient, regardless of their mobility level, has access to the best possible care."
What do the LCD changes mean?
These changes by CMS to Medicare coverage are effective September 1, 2024. The new guidelines expand access to advanced prosthetic knees for more patients, ensuring better technology is available. Changes also include specific requirements for claim processing to improve accuracy and enhance patient care by providing access to state-of-the-art prosthetics and streamlining the claims process. There were no relevant changes compared to the proposed LCD, the coverage criteria are unchanged. However, in the connected Policy Article, the DME MACs have adopted a clarification in the criteria for the K-levels that was proposed by an Interagency Workgroup back in 2017. Overall, it will be easier for prosthetists to qualify patients with accepted and published clinical criteria.
Kenevo: The Only MPK Designed Specifically for Low-Mobility Users
In alignment with Ottobock’s mission to help people maintain and regain their freedom of movement, the global manufacturer saw the need to support the growing population of lower-mobility patients. Ottobock developed the first and only MPK that is designed specifically for this patient group. With a sharp focus on safety, stability, and providing confidence, the Kenevo is unlike any other knee joint on the market today. With this Medicare decision, more patients than ever will have access to the groundbreaking product.
About Ottobock
Ottobock has been a pioneer in the prosthetics and orthotics field, providing innovative solutions that enhance the quality of life for individuals with limb loss. With a rich history of clinical research and technological advancement, Ottobock remains committed to improving patient outcomes and accessibility to cutting-edge prosthetic care.
For more information on Ottobock’s ongoing initiatives and updates on the Medicare LCD changes, please visit shop.ottobock.us.
Ottobock North America Champions Freedom of Movement Through Limb Loss & Limb Difference Awareness Month Initiatives
April 1, 2024 - Austin, TX - During Limb Loss and Limb Difference Awareness Month, Ottobock North America reaffirms its commitment to supporting the limb loss and limb difference community not just during April, but every day of the year. This year, Ottobock is proud to announce the launch of several groundbreaking initiatives aimed at promoting mobility, advocacy, and access to prosthetic and orthotic devices.
Running & Mobility Clinics:
As part of their ongoing dedication to promoting freedom of movement, Ottobock North America will host a series of Running and Mobility clinics across North America. The series kicks off with a Full Circle Movement event in Austin, TX, scheduled for April 20, 2024. The event is designed for individuals of all mobility levels and will offer a supportive environment for participants to explore movement and healing. Attendees will have the opportunity to experience Ottobock’s cutting-edge running-specific prosthetic devices.
Ottobock is also hosting Running Clinics that are designed for more advanced adaptive athletes and will take place over three days and provide intensive coaching from Paralympic athletes. These clinics aim to equip participants with the skills and tools needed to enhance their athletic abilities.
Advocacy Efforts:
In alignment with the 28 by 28 movement, Ottobock North America is joining forces with organizations such as Wiggle Your Toes, AOPA, and the Amputee Coalition to advocate for expanded coverage for orthotic and prosthetic care in 28 states by the 2028 Paralympic Games in Los Angeles, CA. The company recognizes the importance of legislative change in ensuring that individuals with limb loss and limb difference have access to the devices they need to lead active and fulfilling lives.
Aaron Holm, Manager of Consumer Marketing & Engagement and a member of the limb loss community, shares that "Ottobock's mission extends far beyond prosthetics, orthotics, and clinical care. Whether it be continuous legislative advocacy on both the state and federal levels or our stewardship programs, we champion inclusive mobility for all."
#FreedomOfMovement Campaign:
Throughout Limb Loss & Limb Difference Awareness Month, Ottobock North America will highlight the #FreedomOfMovement campaign, emphasizing the importance of mobility for individuals touched by limb loss or limb difference. By empowering individuals with access to prosthetic and orthotic devices, Ottobock aims to celebrate the strength and resilience of the limb loss community.
“At Ottobock, we believe that movement is a fundamental human right. Through our Running & Mobility Clinics and advocacy efforts, we are committed to breaking down barriers and ensuring that individuals with limb loss or limb difference can live life to the fullest,” says Ottobock Head of Government/Medical Affairs & Future Development Scott Schneider.
Get Involved!
Individuals interested in participating in Ottobock North America’s Running & Mobility Clinics or supporting advocacy efforts can find more information by visiting Ottobock.com or by following Ottobock on social media: @ottobock_northamerica
About Ottobock North America
Ottobock North America is a leading provider of prosthetic and orthotic solutions, dedicated to empowering individuals with limb loss or limb difference to pursue their passions and live actively. With a commitment to innovation and excellence, Ottobock strives to enhance the lives of people living with limb loss or limb difference by delivering cutting-edge products and comprehensive support.
For Media inquiries, please contact:
Melissa Langley, Marketing Communications
melissa.langley@ottobock.com
Ottobock at AOPA 2023
Austin, TX (September 1, 2023) – We’re so excited to be back together for AOPA 2023 in Indianapolis, IN from September 6 – 9! As we focus on the message of “driving innovation,” Ottobock has plenty of plans in store to connect with our O&P community at the largest industry event of the year. Please see below for an outline of events, education, and more where you can find Ottobock.
The Ottobock booth will be located at Booth 701, you can’t miss us! Let’s dive into everything going on at AOPA 2023…
2023 Thranhardt Award
The recipients of the 2023 Howard R. Thranhardt Award are Ottobock’s Andreas Kannenberg, MD (GER), PhD, alongside Shane Wurdeman, PhD, CP, FAAOP (D), and Matt Wernke, PhD. The Award has become one of the most distinguished honors in the orthotics and prosthetics profession, annually recognizing the strength in clinical research. We’re thrilled to celebrate these brilliant minds for their research.
Join us during the General Session at 8 a.m. on Friday, September 8 date at to honor the Thranhardt Award recipients at AOPA 2023.
Manufacturer’s Workshop: Making Clinically Relevant Decisions While Bundling Ottobock Componentry
Wednesday, September 6 | 2:00 p.m. – 4:00 p.m. | Presented by Cale Konetchy CP, LP
This course will present attendees with a review of the Ottobock lower extremity product portfolio for K2 and K3 transtibial and transfemoral amputees. A presentation will provide indications, contraindications, and relevant research to support the use of individual components. The presentation will be followed by a demonstration of the newly launched feet from Ottobock.
Manufacturer’s Workshop: Dynion – Elevating Mechanical Knee Stability & Mobility
Wednesday, September 6 | 4:30 p.m. – 5:30 p.m. | Presented by Cale Konetchy CP, LP
Course attendees will learn about the features, functions, and unique design of the first waterproof default stance mechanical knee from Ottobock. A demonstration of the Dynion Knee will provide attendees an opportunity to witness how Ottobock is elevating mechanical knee stability and mobility for above knee amputees.
MyCRO Band Poster Presentation
Thursday, September 7 | 5:00 p.m. – 6:30 p.m. | JP Pineda, CPO
Product Preview Theater: Advancing to New Heights with the Taleo Adjust from Ottobock
Friday, September 8 | 11:30 a.m. – 12 p.m. | Presented by Ariel Cabana, CPO
O&P Digital Showcase: MyCRO Band
Friday, September 8 | 12:00 p.m. – 2 p.m. | Presented by JP Pineda, CPO, Blake Norquist, CO, LO, OTR/L, and Scott Weber of Ottobock
Microprocessor Stance and Swing Control Orthoses Are a Milestone for Patients with Lower Limb Paresis Dependent on Knee-ankle-foot Orthoses
Friday, September 8 | 2:00 p.m. – 3:30 p.m. |Presented by Dr. Andreas Kannenberg, MD, PhD & more
Special events & More:
- Every day at AOPA, anyone can swing by the Ottobock booth to test their skills at the Pit Crew contest, where we will see just how fast you can use the Quickchange Adapter. The winner with the fastest hands will win an exclusive Ottobock swag bag! One winner per day.
- On Thursday, September 7 and Friday, September 8, Ottobock is welcoming attendees to the booth with a coffee and tea bar in the morning to get the day started right.
- 2023 Sam E. Hamontree Award: Show your support by attending and voting for nominees on Thursday, September 7 from 3:45 p.m. – 4:30 p.m. Ottobock’s own Gerry Stark is in the running this year!
- 2023 Hans Georg Näder Digital Education Award: Let’s join together and see the future of O&P with this award dedicated to what’s next in the O&P world and the continuous digital innovations that improve patient care.
Additionally, AllClaim by Ottobock is in Booth 801 to tell attendees more about the latest updates on their billing solutions and services.
General, Business, and Prosthetics Sessions:
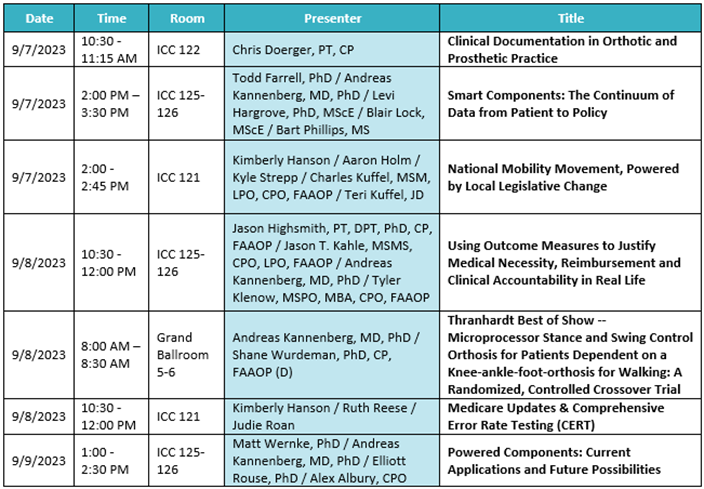
Ottobock Free Papers:
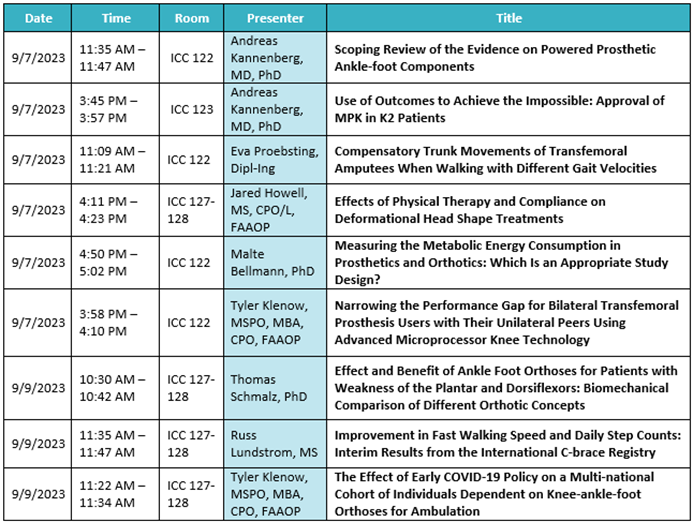
Product highlights:
- C-Leg 4
- Varos Socket & Liner
- WalkOn Lateral / WalkOn Reaction Lateral
- C-Brace & Market Access Updates
- MyCRO Band Cranial Helmet
- Dynion Mechanical Knee
- Quickchange Adapter
- Taleo Adjust
Where else you can find Ottobock...
- AOPA Tailgate Party – Wednesday, September 6, 5:30 p.m. – 7:30 p.m. in Exhibitor Hall
- AOPA Happy Hour – Thursday, September 7, 5:00 p.m. – 6:30 p.m. in Exhibitor Hall
- Professional Women in O&P Luncheon – Friday, September 8, 12:00 p.m. – 1:30 p.m.
We’re looking forward to spending time with so many of our trusted partners!
UPDATE: C-Brace Market Access
Austin, TX (May 22, 2023) – We're so excited to share that as of October 1, 2022, the Center for Medicare and Medicaid Services (CMS) updated the L2006 fee schedule, allowing for wider access to the C-Brace than ever before.

Team Ottobock has been hard at work over the past several years to increase access to the innovative and life-changing C-Brace. We can't wait to see how many more people are able to regain their freedom of movement with this latest reimbursement news.
What does this mean for you?
- C-Brace is on the CMS fee schedule under L2006
- There will be better coverage with private payers
- Favorable reimbursement for C-Brace is now available
How can I get my patients the C-brace?
- Identify your patient as a candidate
- Request a trial
- Partner with Ottobock's dedicated Reimbursement team on the C-Brace journey
Click here for more information on all things C-Brace!
Ottobock Embraces Next-Generation Leadership with the Appointment of Aideen Curran as Director of Professional and Clinical Services
Austin, TX (March 7, 2022) – With changing demographics, the rise of digital technologies, and sharing industry best practices, Ottobock announces the appointment of a dynamic, next-generation leader to take charge of one of the most integral roles in the orthotic and prosthetic industry. Signifying a shift in the way Ottobock approaches education, training, and clinical support, Aideen Curran has been named the Director of Professional and Clinical Services (PCS), Ottobock North America.

“My vision will always be a patient-first approach to education and training. The patient is at the heart of everything we do as a CPO, and my purpose in this role is to leverage the strengths of this world-class specialist team to transform how we deliver education. Our goal as a department is to achieve exceptional patient outcomes by empowering and enriching the lives of the patients we serve in partnership with our customers,” said Aideen Curran, Director of Professional and Clinical Services, Ottobock North America. “I am honored to lead such an exemplary team who are fundamentally shaping the future of our industry through their thoughtful leadership, and though this will be my biggest and most exciting challenge to date, I am looking forward to the positive advancements we will make for our customers and their patients.”
Innovation is fueling the next generation in the orthotic and prosthetic industry. Evolving expectations represent new ways of thinking, engaging, receiving educational information, learning from peers and practices around the world, and opening new doors with diversity of background, identity, and experience. The next generation is more comfortable embracing the latest digital technologies and innovations, such as 3D printing and digital scanning, as they reconsider traditional approaches. They are also interested in breaking down geographical borders to learn best practices from a global perspective.
“Aideen is a strong leader who not only adds diversity in a critical role in the O&P industry, but she also demonstrates an intense focus on the customer, the patient, and their outcomes. She earned her promotion with skill, acumen, hard work, and a fearless attitude,” said Chris Nolan, General Manager, Ottobock. “She is the right person at the right time to provide the leadership that is needed to take PCS to the next level. Customers, who all look to Ottobock for first-class training, education, and product support, will be very happy with the way Aideen rolls out her vision for consistency in delivering education and training.”
Ottobock is committed to unlocking more value for direct customers and distribution partners. Customers need to deliver products at a faster pace than ever, yet without compromise. The integrated digital future is now, and the company’s PCS team is evolving to stay ahead of the curve, practicing continuous improvement. Having already been providing digital education for over a decade, the company will continue to lead the digital accessibility of education, expanding and fortifying its position.
“It’s an exciting time for Aideen to take over the leadership of the orthotic and prosthetic industry’s largest clinical services and technical support team in her new role,” said Mark Edwards, who has been the head of Ottobock’s clinical services department for more than a decade and is assuming a new role as Senior Advisor until he retires. “Aideen is a go-getter who is highly motivating and forward-thinking. She brings with her the global experience to maximize the value of integrating new approaches and different methods in the North American market as part of a refresh.”
Aideen is the former Education Manager at Ottobock NA and joined Ottobock’s U.S. Professional Clinical Services team after seven years at Ottobock Australia, where she was responsible for the Ottobock Academy, a dedicated training facility for CPOs and technicians across Australia and New Zealand. To expand her clinical and technical experience, she trained extensively at Ottobock’s Global Academy in Germany and gained significant global perspective across Ottobock subsidiaries and patient care networks in Europe and the Asia Pacific. She earned her degree in Prosthetics and Orthotics at La Trobe University Melbourne, Australia.
About Ottobock
Founded in 1919, Ottobock develops “wearable human bionics” – medical technology products for people with limited mobility in the fields of Prosthetics, Orthotics, and Wheelchairs. Ottobock's mission is to improve their quality of life and increase health economic benefits. Ottobock has been supporting the Paralympic Games with its technical expertise since 1988. For more information, go to: www.ottobock.com.
Ottobock Introduces New and Improved Extended Warranty Options for Select Upper Limb Devices
Austin, TX (January 13, 2022) – We're excited to announce that Extended Warranty options for our bebionic hands, Michelangelo hands, and DynamicArms have been enhanced with expanded coverage. These new extended warranty options are called Ottobock Care Packages:
- New! Accidental Damage Coverage
Care Packages include accidental mechanical damage caused by the user during proper use. For example, a broken finger caused by applying excessive force. - Faster, more efficient service
With an Ottobock Care Package, repairs can be handled right away, without cost estimates or approval delays. - Premium Coverage for Premium Products
Care Packages are available for the bebionic hand, Michelangelo hand, and DynamicArm. Loaner Units are provided at no-charge during any needed maintenance or repairs.
Questions? Download our flyer.
Ottobock Stewardship Committee supports 35 nonprofit organizations in 2021
Austin, TX (January 4, 2022) – Ottobock’s Stewardship Committee delivered on the commitment to supporting nonprofit organizations who align with Ottobock’s mission through charitable donations. Every year, this committee of cross-functional employees works together and within the greater community to foster real change and movement in the amputee and limb difference community – and beyond.
“When I started my nonprofit in 2008, Ottobock was the first organization to provide sponsorship/funding. It is a privilege for me to now be a part of the team that provides support to the nonprofit organizations that align with Ottobock’s mission and values – many of which are small startups similar to where my organization began,” said Aaron Holm, Manager of Consumer Engagement at Ottobock. “We applaud the initiatives of the leaders, volunteers, and communities that surround the organizations the Stewardship Committee supports. On behalf of myself and the Ottobock Stewardship team, thank you for your efforts and contributions.”
From stroke recovery to diabetes research to pediatric prosthetics, and everything in between, Ottobock’s Stewardship Committee and their donations branched to many touchpoints of the community they serve.
“Because of Ottobock’s generous support, Jordan Thomas Foundation is now serving 77 kids living with limb loss around the country. 77 kids now have access to their childhoods that had been denied them,” said Jordan Thomas, Co-Founder of the Jordan Thomas Foundation. “We’re extremely grateful for this generosity that allows us to do the life-changing work we do at Jordan Thomas Foundation.”
In support of Ottobock’s mission to help people maintain or regain their freedom of movement, the Stewardship Committee donated for the following 35 organizations across the U.S. and Canada:
- Abled Amputees: Bridging the financial gap for outpatient physical and occupational therapy for amputees.
- Accessible Prosthetics Initiative: Student-driven startup nonprofit helping amputees through community outreach and education.
- ACPOC: Pediatric initiative awarding clinical education and research to raise the standard of prosthetic care for children.
- Adaptively Abled: Support, educate, and inspire those living with limb loss and limb difference through educational programs and adaptive fitness/wellness efforts.
- Amputee Blade Runners: Provides free running devices for amputees.
- Amputee Coalition: Empowering people affected by limb loss to achieve their full potential through education, support, and advocacy, and promote limb loss prevention.
- Andy Roddick Foundation: Expanding opportunities for young people to learn, thrive, and succeed.
- Angel City Foundation: Sports programming for kids, adults, and veterans with physical disabilities or visual impairments.
- Arizona Disabled Sports: Adaptive sports programming for kids and adults with physical disabilities.
- Austin Speech Labs: Improving lives post-stroke.
- Choosing to See: Build awareness and create connections between sighted and visually impaired communities.
- Collège Montmorency (Quebec): Bursaries to Prosthetic and Orthotic program students.
- The Eastern Amputee Golf Association: Organize and conduct amputee golfing events.
- Enhancing Skills for Life: Educate, empower, and connect with those living with bilateral upper limb loss.
- Hope Faith Ministries: Horizon O&P fundraiser for shoe and sock drive for local communities; HFM works to alleviate homelessness and poverty in Kansas City by providing basic necessities and assistance and programs to help empower individuals.
- Husome Strong: Helping the amputee community through peer support, advocacy, and financial assistance.
- JDRF: Leading the fight against Type 1 diabetes by advocating for policies that accelerate access to new therapies and provide a support network.
- Jordan Thomas Foundation: Provides children with limb loss the prostheses they need throughout their growing years while serving as a caring resource.
- Learning Links: Creates a place where anyone can learn and enjoy the sport of golf, regardless of ability, skill, or age.
- Limb Lab Foundation: Providing low to no cost, highly functional prosthetic and orthotic devices to help people in need.
- Limbs For Life Foundation: Global nonprofit dedicated to providing fully-functional prosthetic care for individuals who could not otherwise afford it.
- Mary Free Bed: Largest nonprofit rehabilitation system in the country to provide resources for short and long-term care.
- Move for Jenn Foundation: Offer grants in the form of activewear prosthetics to those who have limb loss or other diseases.
- National Abilities Center: Empower individuals of all abilities by building self-esteem, confidence, and lifetime skills through sports, recreation, and education.
- OAPO Canada: Aims to improve the quality of life for people who may benefit from the rehabilitation practice of prosthetic, orthotic, mobility, and assistive technology.
- OPAF: Provides adaptive recreation opportunities for those with physical and mobility challenges.
- Range of Motion Project: Provides high quality prosthetic care in underserved populations to enhance mobility and unlock human potential.
- REA Foundation: Supports the largest rehabilitation center for physical (motor, sensory and language) disabilities in Quebec.
- Roger C. Peace Rehab Hospital: Specialize in medical treatment, rehabilitation and research, and providing inpatient and outpatient programs for patients with spinal cord injury, brain injury, stroke, amputation, and other neurological conditions.
- SFM Foundation: Scholarships to children of workers disabled or killed in work-related accidents.
- Steps of Faith: Turning prosthetics into possibilities for uninsured amputees.
- Team Catapult: Catapult physically challenged individuals over adversity and into the world of endurance sports.
- Theresa Tracy Strive to Survive: Create awareness around pancreatic cancer and fund research for early detection, testing, and treatment of pancreatic cancer.
- Wellspan: Integrated health system that serves the communities of Central PA and northern MD.
- Wiggle Your Toes: Helping the limb loss and difference community heal, recover, and flourish.
The Stewardship Committee is looking forward to opportunities to give back to the community in 2022 and beyond.
Ottobock Rings in the New Year with Expanded Warranty Coverage for bebionic Hands
Austin, TX (January 1, 2022) – At Ottobock, we strive to provide resources that allow Clinicians to focus on what’s most important: fitting the best hand for their patients. We recognize that the cost of ownership is a consideration when selecting a terminal device. To further provide best in class service and support, we are very pleased to announce Expanded Warranty Coverage for bebionic hands in North America.
Effective January 1, 2022, warranty coverage for bebionic hands will now include replacement of the following wear and tear parts:
- Palm Gaiter – Small hands
- Thumb Gaiter – Small hands
- Gaiter – Medium Hands
- Distal Finger Palps – Small Hands
- Distal Finger Palps (includes tip replacement) – Medium Hands
- Clevis Links
Coverage of these wear and tear parts will greatly reduce bebionic service and repair costs for our customers. Additionally, it expedites service by eliminating estimates and approval turnaround.
Please note, bebionic hands come with a standard 2-year manufacturer's warranty. Extended warranty packages up to 5 years are available for purchase. The bebionic hand must be under warranty for coverage of the wear and tear parts noted above. Our optional bebionic gloves are excluded from wear and tear coverage.
PDAC Verification Update: Ottobock bebionic Hand
Austin, TX (November 23rd, 2021) – The following product has received coding verification effective November 18, 2020, and must be billed to Medicare with the stated HCPCS codes.
- Product: Ottobock bebionic Hand PDAC Verified Coding: L6880
As a reminder, we are maintaining a portal section that includes all Ottobock multi-articulating hands, feet, and microprocessor knees with PDAC verified coding. This document indicates the effective date, PDAC verified codes, and previously recommended coding for each product. Additionally, you will find a link to the PDAC verification letter.
WalkOn Flex Junior Launches for Children with Drop Foot
Austin, TX (October 18th, 2021) – Ottobock is excited to introduce the latest addition to the WalkOn family, the 28U22 WalkOn Flex Junior. Utilizing the same flexible design as the WalkOn Flex, the WalkOn Flex Junior is designed for children with mild to moderate drop foot, giving them active, dynamic gait and stability as they walk.
Benefits at a Glance:
- Dynamic design and advanced carbon fiber construction for excellent energy storage and return during gait cycle
- Medial guidance of the longitudinal arch prevents supination in solid footwear
- Spiral design provides greater flexibility at heel strike
- Trimmable footplates easily shaped with scissors, often requires only one office visit
- New and improved calf band with phase change material (temperature regulative)
- No activity or weight restrictions
- 60-day money back guarantee and 12-month warranty (tested for one year durability)
- Packaged in a bright, kid-friendly sports bag
Walk more naturally.
To learn more, please visit our WalkOn family page.
Ottobock at AOPA 2021
Boston, MA, September 7, 2021 – At this year’s AOPA Conference from September 9 - 11, Ottobock is hosting multiple major activities for O&P professionals to observe, learn, network and more. With a focus on leading the digital future of O&P, see below for all educational content, live demos, and first looks. Learn more below.
Varos + Kenevo + Kintrol Workshop: Augmented stability integrating Kenevo MPK, Kintrol hydraulic foot, and Varos socket technology by Ottobock
Studies show that lower mobility users show remarkable benefits and outcomes, if they are fit with a prosthesis immediately following amputation. The innovative Varos socket from Ottobock helps clinicians fit patients quickly and avoid the more time-consuming processes of traditional impression-taking and fabrication methods. In combination with the Kenevo microprocessor knee and Kintrol hydraulic foot, clinicians can offer a wider array of options for greater stability, enhanced mobility, adjustability and comfort in the fitting process. Discussion will focus on patient selection, fabrication processes, and clinical techniques to optimize patient experience, and function as well as a variety of other clinical tools from Ottobock.
Join this workshop on Thursday, September 9 from 8:00 a.m. to 10:00 a.m. EST at Sheraton Boston Hotel, Room Beacon D.
Digital to Walk Series PART I: Building a complete leg prosthesis using Ottobock’s new scan-to-print technology
In this workshop we will begin by reviewing the Custom 4U liner scanner to create a custom liner. Following this, we will introduce you to the new iFab (individualized fabrication) Suite, which consists of iFab EasyScan, the Ottobock 3D-scanning solution, and web-based modelling in the iFab Customer Center. We will demonstrate a scan of a transtibial patient model using the new Ottobock-driven Intel hand-held scanner and explore the features of the EasyScan app to create patient profiles for 3D scans of custom liners, TT diagnostic sockets, and MyFIt TT 3D-printed socket. After modifying the socket via iFab Design, we will complete the process with a live dynamic walk on the MyFit TT 3D-printed socket. To enhance the cost-effectiveness of building a complete leg prosthesis, we will finish the session by discussing new Lower Limb Bundles. Ottobock is excited to demonstrate the first all-inclusive scanning, modelling, and 3D-printing technology specifically designed for the O&P industry. This end-to-end solution significantly lowers the entry barriers to digitalization to improve the efficiency of the care pathway for your patients.
Join Part I of the workshop on Thursday, September 9 from 10:30 a.m. to 12:30 p.m. EST at Sheraton Boston Hotel, Room Beacon D.
Digital to Walk Series PART II: Using the latest digital tools and products from Ottobock to optimize outcomes for orthotic and prosthetic patients
Digital tools are revolutionizing treatment pathways within the field of orthotics and prosthetics. These tools help clinicians optimize alignment and evaluate dynamic composite feet, as well as microprocessor prosthetic and orthotic designs with precision. In addition to the 3D LASAR Posture, Ottobock is pleased to present a new mobile gait analysis system, the Bionic Pro, using onboard positional sensors for the 10m walk test and foot drop analysis. This new system will be used with three clinical patient cases involving the new Empower microprocessor ankle, the Taleo Vertical Shock and new Taleo Side Flex feet and the C-Brace microprocessor orthosis.
A significant added benefit of these clinical tools is to support documentation and reimbursement to provide higher level clinical notes to share outcomes and provide quantifiable data to demonstrate value. Using the objective data from the Bionic Pro, and subjective information during initial evaluation, these case examples will be used to demonstrate the basics of a good clinical note and effective documentation.
Join Part II of the workshop on Thursday, September 9 from 1:30 p.m. to 3:30 p.m. EST at Sheraton Boston Hotel, Room Beacon D.
If you are unable to attend in person and want to dive into Ottobock educational workshops, they will be available virtually on our YouTube channel after the conference ends.
Additionally, Ottobock is hosting multiple live demos, first looks, and more at Booth 415 at the trade show. Swing by the booth to see:
- Live demo of our Easy Scan process for the new 3D-printed MyFit TT socket
- Live demo of Bionic Pro, digital gait analysis tool to help you provide great outcomes and data for payers
- A sneak peek at MyCRO Band, the world's first FDA-cleared, 3D-printed cranial remolding band
- Additions to our foot portfolio, include Taleo Side Flex and the Kintrol hydraulic foot
Ottobock is thrilled to be a part of AOPA for another year, and even more thrilled that we’re back in person with colleagues from around the country. If we’ve learned anything since March 2020, it is that we truly are stronger together. It’s an honor to celebrate that this year.
Not attending AOPA in person? Check out the digital future of Ottobock right here.
Landmark clinical trial of seniors with limb loss aims to increase access to advanced prosthetic technology
Austin, TX (August 26th, 2021) – Hanger, Inc. and Ottobock today announced they have joined resources to conduct a landmark five-year clinical study to collect data around what potential health benefits microprocessor-controlled knee (MPK) technologies provide people 65 and older. This endeavor would establish evidence that could expand and support new coverage policies, offering greater access to seniors with above-knee limb loss.
The Hanger Institute for Clinical Research and Education and Ottobock are partnering with Hanger Clinic prosthetists around the country to conduct this landmark prospective randomized trial. ASCENT K2 (ASsessing outComes with microprocEssor kNee uTilization in a K2 population) will measure the short and long-term effects of MPK use in K2-level community ambulators, meaning people who can navigate low-level environmental barriers like stairs and curbs. Specific data points measured will include health-related quality of life, participation in society and activities, fall rates, and participants’ fear of falling.
ASCENT K2 began enrolling test subjects and fit its first MPK recipient in July. By the end of March 2022, 100 Hanger Clinic patients will be enrolled in the study. Half the patients will be randomized into a group fit with an Ottobock MPK, an advanced prosthetic technology typically only covered for higher-level K3 or K4 ambulators. The other half will form a control group using mechanical, non-MPK knees, the current standard for K2 ambulators. Participants will be assessed periodically throughout their first 12 months in the study, with initial analyses published at the one-year mark, and then annually for five years.
“We are delighted to leverage our collaborative research capabilities and clinical expertise to gather much-needed data about the growing population of seniors with lower limb amputations,” stated Dr. James Campbell, Chief Clinical Officer of the Hanger Institute for Clinical Research and Education. “This important research is designed to help ensure people living with limb loss have coverage for medically necessary and clinically appropriate technology, which will, in turn, enable them to participate more fully in their communities and enjoy a higher quality of life.”
“Ottobock has been working on the generation of scientific evidence for the benefits of MPK in K2 patients for 15 years now with several clinical studies conducted at academic research centers with only limited access to this patient population. We are very pleased that our partnership with Hanger Clinic enables our two companies now to run the biggest interventional study yet in the real-life environment of prosthetic clinics that should eventually compel the healthcare payer community of the benefits that MPK deliver to limited community ambulators,” said Dr. Andreas Hahn, VP Clinical Research & Services, Otto Bock Healthcare Products GmbH, Vienna (Austria).
About Hanger, Inc.
Headquartered in Austin, Texas, Hanger, Inc. (NYSE: HNGR) provides comprehensive, outcomes-based orthotic and prosthetic (O&P) services through its Patient Care segment, with approximately 800 Hanger Clinic locations nationwide. Through its Products & Services segment, Hanger distributes branded and private label O&P devices, products and components, and provides rehabilitative solutions. Recognized by Forbes as one of America’s Best Employers for 2021, and rooted in 160 years of clinical excellence and innovation, Hanger is a purpose-driven company with a vision to lead the O&P markets by providing superior patient care, outcomes, services and value, aimed at empowering human potential. For more information on Hanger, visit investor.hanger.com OR news.hanger.com.
About Ottobock
Ottobock develops “wearable human bionics” – medical technology products for people with limited mobility in the fields of prosthetics, neuro-orthotics, human mobility (wheelchairs) and exoskeletons. The company, founded in 1919, also treats patients in its Patient Care division. Ottobock's mission is to improve their quality of life and increase health economic benefits. With the Paexo exoskeletons, Ottobock has transferred its expertise in biomechanics to applications for industry as well since 2012. Subsidiaries in almost 60 countries offer “Made in Germany” quality worldwide and employ more than 8000 people. The international activities of the company are coordinated from the head office in Duderstadt (state of Lower Saxony). Ottobock has been supporting the Paralympic Games with its technical expertise since 1988.
Media Contacts
Krisita Burket, Hanger, Inc.
904-239-4627, kburket@hanger.com
Meghan Williams, Hanger, Inc.
512-777-3701, megwilliams@hanger.com
Cali Solorio, Ottobock
512-470-7119, cali.solorio@ottobock.com
Ottobock Launches the New Kenevo®
Austin, TX (June 7th, 2021) -- Ottobock is excited to announce the launch of the new Kenevo microprocessor knee. Since its launch in 2015, the Kenevo fundamentally changed prosthetics for low to moderately active amputees. The Kenevo is the world’s first and only microprocessor knee designed specifically with this patient group in mind.
The Kenevo is an intuitive, intelligent solution making everyday life safer and easier for its users. The new features and functions build upon the foundation of this life-changing technology, allowing its users to feel more mobile and independent than ever before.
What’s new:
- Donning of the prosthesis while seated thanks to complete flexion of the knee joint
- Automatic switching to lower knee flexion resistance for use of a stationary indoor bicycle
- Convenient charging without removing the foam cover
- Automatic ramp detection with additional support during descent
- User-controlled adjustments to the knee through the Cockpit App
- Now approved for hip disarticulation
- Redesigned inductive charger making it easier for those with hand dexterity challenges
- Wheelchair lock more robust and holds over bumpy terrain
- Smoother stance extension
Suggested Coding Changes for Ottobock Feet
As we continue to evaluate feedback received from our PDAC submissions and additional code language clarification published, we adjusted our suggested codes for the following Ottobock feet as of April 16, 2021. Please note the changes from previously recommended coding.
Product: 1C53 Taleo Low Profile
Previously recommended codes: L5981 + L5986
New Ottobock recommended code: L5981
Product: 1C63 Triton Low Profile
Previously recommended codes: L5981 + L5986
New Ottobock recommended code: L5981
Product: 1C40 C-Walk
Previously recommended codes: L5981 + L5986
New Ottobock recommended code: L5981
The O&P Provider assumes full responsibility for accurate billing of Ottobock products. Ottobock Coding Suggestions are based on reasonable judgment and are not recommended to replace the O&P Provider’s judgment. These recommendations may be subject to revision based on additional information or alpha-numeric system changes.
Because these changes will result in a reduction in reimbursement, Ottobock made pricing concessions in January 2021 for the 1C53 Taleo Low Profile and 1C63 Triton Low Profile to alleviate the potentially negative financial impact on your business.
We believe the Taleo and Triton family of carbon fiber feet deliver the optimal outcomes your patients deserve. We are committed to you and believe this pricing change will enable you to continue fitting Ottobock foot solutions. For detailed information regarding specific pricing changes, please contact your sales representative or log into your Ottobock online account.
Additionally, if you have any coding or reimbursement questions, please contact Ottobock Reimbursement Support. Email us at reimbursement911@ottobock.com, or call us at 800.328.4058.
Suggested coding for Freedom Innovations feet retained by Ottobock
After careful consideration, Ottobock recommends the following codes for the Ottobock Freedom Innovations feet as of April 7, 2021. Please note the changes from previously recommended coding.
Product: Maverick Xtreme
Previously recommended codes: L5987 + L5986
Ottobock recommended code: L5981
Product: Maverick Xtreme AT
Previously recommended codes: L5987 + L5986
Ottobock recommended code: L5981
Product: Renegade AT
Previously recommended codes: L5987 + L5986
Ottobock recommended code: L5981
Product: Renegade LP-AT
Previously recommended codes: L5987 + L5986
Ottobock recommended code: L5981
Product: Silhouette VS
Previously recommended codes: L5987 + L5986
Ottobock recommended code: L5980
Product: Silhouette LP-VS
Previously recommended codes: L5987 + L5986
Ottobock recommended code: L5980
Product: Renegade
Previously recommended codes: L5987
Ottobock recommended code: L5981
Product: Renegade LP
Previously recommended codes: L5987
Ottobock recommended code: L5981
Product: Thrive
Previously recommended codes: L5981 + L5999
Ottobock recommended code: L5981
The O&P Provider assumes full responsibility for accurate billing of Ottobock products. Ottobock Coding Suggestions are based on reasonable judgment and are not recommended to replace the O&P Provider’s judgment. These recommendations may be subject to revision based on additional information or alpha-numeric system changes.
In an effort to offset disruption or inconvenience brought on by this change, please note the suggested alternative PDAC Verified Vertical Shock Feet offered by Ottobock below:
Product: 1C51 Taleo Vertical Shock
PDAC Verified Coding: L5987 + L5986
1C51 Taleo Vertical Shock PDAC Verification Letter
Product: 1C61 Triton Vertical Shock
PDAC Verified Coding: L5987 + L5986
1C61 Triton Vertical Shock PDAC Verification Letter
If you have any questions, please contact Ottobock Reimbursement Support. Email us at reimbursement911@ottobock.com, or call us at 800.328.4058.
Additionally, please contact Ottobock Customer Service or your Sales Representative for new pricing established for the above products.
We will continue to keep you informed as our integration efforts continue. Thank you for your commitment to Ottobock and for trusting us as a partner in helping your patients reach their mobility and activity goals.
Urgent Update: Friday, April 30th Early Shipping Cutoff 3:00 PM CST
Due to the anticipation of traffic delays related to Oaks Day and the Kentucky Derby, UPS has changed our regularly scheduled pickup time on Friday, April 30th for the Louisville, KY logistics center. All priority orders must be received by 3:00 PM CST on Friday, April 30th in order to be shipped same day.
For assistance placing an order, please contact our Customer Service Team.
UPS has informed us that normal operations will resume Monday, May 3rd.
Thank you for your understanding.
-Your Partners in O&P Care
Addition of New Agilium® Forte Orthosis Completes Ottobock’s Line of Orthoses for Knee Osteoarthritis
Austin, TX (April 9, 2021) – We're excited to announce the launch of Agilium Forte, a new rigid knee orthosis designed to provide lasting pain relief for patients with moderate to severe unicompartmental osteoarthritis (OA). Launched March 29, Agilium Forte is the latest addition to our Agilium family of knee OA orthoses developed for everyday use, recreational activities, and sports.
What’s special about Agilium Forte?
- Dynamic Y force strap for effective unloading
- Rigid shells for stabililty and open sleeve design for patient comfort
- Trimmable sleeves reduce inventory to only three sizes
Less Pain. More Life.
The new Agilium Forte extension of the Agilium Family creates a complete product offering for the treatment of knee osteoarthritis. To learn more about our other products in the Agilium Line, please visit our new Agilium Family page.
Ottobock North America Appoints MedTech Industry Leader Marc C. Lundeberg as CEO & Regional President
AUSTIN, Texas (February 1, 2020) – Today, Ottobock and its Board of Directors announced the appointment of Marc C. Lundeberg as the new CEO and Regional President of the company’s North America operation. Effective February 1, 2021, Marc succeeds Brad Ruhl, who will remain on as Non-Executive Chairman until he transitions to retirement after a more than 30-year tenure with Ottobock.
Lundeberg joins Ottobock North America from Amplifon, where he held the position of CEO & President for North America, since May 2017. “After careful consideration, the board concluded that Marc was the right leader to take the reigns from Brad, who has led the region to record profitability and expanded Ottobock’s footprint across the US and Canada,” said Philipp Schulte-Noelle, Ottobock Global CEO. “Marc brings a tremendous amount of expertise and a distinguished track record of strategic, operational, and people leadership to our growth path. I am delighted to welcome Marc to the Ottobock Family.”
“I am thrilled to join a global leader in its field and help Ottobock continue setting new standards in the pursuit of its mission to help people maintain or regain their freedom of movement,” said Lundeberg.
About Ottobock
For more than 100 years, Ottobock has been developing med-tech products and fitting solutions for people with limited mobility in the areas of Prosthetics, Orthotics and Human Mobility. The company’s international activities are coordinated from the head office in Duderstadt, Germany. Ottobock has been investing, employing, researching and developing in North America since 1958 for the benefit of people with impaired mobility. Subsidiaries in 59 countries offer “Made by Ottobock” quality worldwide and employ more than 8,000 people.
Media Contact
Ottobock North America
Cali Solorio
Director of Marketing & Communications
cali.solorio@ottobock.com
NEW! Competitive Bidding Resource Center
Round 2021 of the DMEPOS Competitive Bidding Program kicked off this year and will remain in effect through December 31, 2023. Medicare beneficiaries needing off-the-shelf (OTS) spine and knee orthoses listed in the program in the Competitive Bidding Areas (CBAs) must obtain them from qualified suppliers that have been awarded contracts from the Centers for Medicare & Medicaid Services (CMS).
We are excited to share our Competitive Bidding Resource Center to help you quickly locate information about the program.
Resource Center:
- Search OTS spine and knee orthoses by HCPCS code
- Visit individual product pages to learn more
- Purchase products directly from our website
- Download key Competitive Bidding documents
If you did not win a bid, you can still utilize the Resource Center to download information and help refer patients to a qualified suppler in your area.
For questions or support, don’t hesitate to reach out to Ottobock’s Reimbursement team anytime at ottobockreimbursement@ottobock.com.
Ottobock Stewardship Committee supports 20 nonprofit organizations in 2020
AUSTIN, Texas (February 1, 2020) – Despite the challenges of last year, Ottobock’s Stewardship Committee delivered on our commitment to supporting nonprofit organizations who share in our mission’s goal through charitable donations.
"The Stewardship program provides support to help fuel nonprofit organizations and the passionate people who manage them," said Aaron Holm, Manager, Consumer Engagement at Ottobock. "These organizations and the people who manage them become partners of Ottobock – working together toward a common goal, paying it forward within the community we serve."
Ottobock’s mission is to help people maintain or regain their freedom of movement. In 2020, we contributed donations to the following nonprofit organizations across the United States and Canada:
- Amputee Coalition: Empowering people affected by limb loss to achieve their full potential through education, support, and advocacy, and promote limb loss prevention.
- Andy Roddick Foundation: Expanding opportunities for young people to learn, thrive, and succeed.
- Angel City Sports: Sports programming for kids, adults, and veterans with physical disabilities or visual impairments.
- Arizona Disabled Sports: Creating opportunities that empower through sports and recreation.
- Austin Speech Labs: Improving lives post-stroke.
- Challenge Alaska: Improving lives of people with disabilities through adaptive sports, therapeutic recreation, and education.
- Collège Mérici (Quebec): Bursaries to Prosthetic and Orthotic program students.
- Collège Montmorency (Quebec): Bursaries to Prosthetic and Orthotic program students.
- Husome Strong: Helping the amputee community through peer support, advocacy, and financial assistance.
- ISPO / OAPO / AGM Canada: These organizations aim to improve the quality of life for people who may benefit from the rehabilitation practice of prosthetic, orthotic, mobility, and assistive technology.
- Jordan Thomas Foundation: Provides children with limb loss the prostheses they need throughout their growing years while serving as a caring resource.
- Learning Links: Creates a place where anyone can learn and enjoy the sport of golf, regardless of ability, skill, or age.
- MSOPP: Excel the professional and ethical standards of O&P and pedorthic practitioners.
- NAAOP Fellowship: O&P policy, advocacy, and betterment of community.
- Paratough Cup: Ensure Canadians with a disability can have access to sport, from their communities all the way to the world stage.
- Range of Motion Project: Provides high quality prosthetic care in underserved populations to enhance mobility and unlock human potential.
- SFM Foundation: Scholarships to children of workers disabled or killed in work-related accidents.
- Steps of Faith: Turning prosthetics into possibilities for uninsured amputees.
- Wiggle Your Toes: Helping the limb loss and difference community heal, recover, and flourish.
Ottobock contributed to help fund post-secondary scholarships to organizations like SFM Foundation, which awards young people from families affected by workplace tragedies to pursue higher education. "We have been able to award 187 scholarships to deserving students," said Linda Williams, President of SFM Foundation. "Thank you for generously supporting educational futures for young adults."
To request a donation for a nonprofit organization in 2021, please contact your local Ottobock sales representative for more details.
For any questions, contact stewardship@ottobock.com. We appreciate your involvement in helping us support our local communities and partners in 2021.
Media Contacts
Ottobock North America
Aaron Holm
Manager, Consumer Engagement
aaron.holm@ottobock.com
512-470-7100
Positive Future for Freedom Innovations Set
Transaction with Proteor approved by U.S. Federal Trade Commission and closed today
Austin / Duderstadt (December 11, 2020) – Ottobock, the internationally operating medical technology company, welcomes the U.S. Federal Trade Commission’s (FTC) final decision on the future of Ottobock’s prior acquisition of Freedom Innovations (FI). Today, Ottobock has formally closed a transaction with French orthopaedic technology specialist Proteor SAS, which has acquired significant assets of Freedom Innovations. The corresponding purchase agreements were previously signed in September, but subject to FTC approval.
"For all parties involved and especially for the employees of Freedom Innovations, the final solution is good news after several years of uncertainty and sets the future for Freedom Innovations under the roof of both Proteor and Ottobock,” says Philipp Schulte-Noelle, CEO of Ottobock. "I am especially grateful for the very constructive dialogue with the FTC in recent months and the engagement of Proteor."
“I would like to thank all Freedom Innovations employees for their continued dedication, focus, hard work, and professionalism during this unpredictable time. We look forward to coming together as one family,” says Edouard Archambeaud, member of the Board of Directors of Proteor. “I would also like to thank Ottobock for working diligently towards a successful outcome and the FTC for their vote of confidence in our acquisition.”
The transaction allows Ottobock to strengthen its product portfolio in the field of prosthetic feet as it will retain certain Freedom Innovations foot products. Specifically, the transaction contemplates Ottobock keeping the Kintrol K2 hydraulic foot-ankle prosthesis, the Maverick fiberglass product family and other carbon feet expanding the company’s product portfolio.
Proteor will acquire the microprocessor-controlled knee Plié3, the Kinnex and Kinterra ankles, certain prosthetic feet and other infrastructure from FI. Proteor will also receive the intellectual property rights related to these transferred products and the Freedom Innovations brand and take over the R&D activities of FI.
With this transaction, Ottobock satisfies FTC’s order to divest certain assets from Freedom Innovations. As background: Ottobock acquired FI in September 2017 with the goal of strengthening the prosthetic product choices and technology for the benefit of amputees and clinicians. The FTC filed a complaint against the acquisition and prohibited the integration of FI by Ottobock. An application for a final approval was filed with the FTC on 9 October 2020. This was followed by a 30-day public comment period. This period ended without any significant objections. The final decision was made by the FTC's highest body, the FTC Commissioners on 30 November 2020.
To consolidate ordering and technical support during this transitional phase, please use the following link for details about the split of Freedom products between Ottobock and Proteor:
Guide: Freedom Innovations Transition to Ottobock & Proteor
Contact person for media enquiries:
Europe
Vice President Investor Relations & Corporate Communications
Mark C. Schneider
Ottobock SE & Co. KGaA, Prenzlauer Allee 242, 10405 Berlin, Germany
Phone: +49 30 398 206 222
Mobile: +49 151 146 591 35
E-mail: MarkC.Schneider@ottobock.de
North America
Director of Marketing & Communications
Cali Solorio
Ottobock North America
Phone: +1 512 806 2655
Mobile: +1 512 470 7119
Email: Cali.Solorio@ottobock.com
PDAC Verification Update: 1C51 Taleo Vertical Shock, 1C52 Taleo Harmony and 1C62 Triton Harmony
The following products have received coding verification effective November 21, 2020, and must be billed to Medicare with the stated HCPCS codes.
- Product: 1C51 Taleo Vertical Shock
PDAC Verified Coding: L5987+L5986
Previous Codes: L5987+L5984 - 1C51 Taleo Vertical Shock PDAC Verification Letter
- Product: 1C52 Taleo Harmony
PDAC Verified Coding: L5987+L5986+L5781
Previous Code: L5987+L5984+L5781 - 1C52 Taleo Harmony PDAC Verification Letter
- Product: 1C62 Triton Harmony
PDAC Verified Coding: L5987+L5986+L5781
Previous Code: NO CHANGE IN CODING - 1C62 Triton Harmony PDAC Verification Letter
As a reminder, we are maintaining a portal section that includes all Ottobock feet and microprocessor knees with PDAC verified coding. This document indicates the effective date, PDAC verified codes, and previously recommended coding for each product. Additionally, you will find a link to the PDAC verification letter.
PDAC Verification Update: Additional Prosthetic Feet
1E58 Axtion DP, 1E90 Sprinter, 1E91 Runner, 1E93 Runner Jr, and 1E2 ProCarve Foot
The following product has received coding verification effective November 19, 2020, and must be billed to Medicare with the stated HCPCS codes.
- Product: 1E58 Axtion DP
PDAC Verified Coding: L5980
Previous Codes: L5980+L5986 - Product: 1E90 Sprinter
PDAC Verified Coding: L5999
Previous Code: L5980 - Product: 1E91 Runner
PDAC Verified Coding: L5999
Previous Code: L5980 - Product: 1E93 Runner Junior
PDAC Verified Coding: L5999
Previous Code: L5980 - Product: 1E2 ProCarve Foot
PDAC Verified Coding: L5999
Previous Code: L5987
As a reminder, we are maintaining a portal section that includes all Ottobock feet and microprocessor knees with PDAC verified coding. This document indicates the effective date, PDAC verified codes, and previously recommended coding for each product. Additionally, you will find a link to the PDAC verification letter.
PDAC Verification Update
The following product has received coding verification effective November 18, 2020, and must be billed to Medicare with the stated HCPCS codes.
- Product: 1E95 Challenger
PDAC Verified Coding: L5981
Previous Code: L5987
As a reminder, we are maintaining a portal section that includes all Ottobock feet and microprocessor knees with PDAC verified coding. This document indicates the effective date, PDAC verified codes, and previously recommended coding for each product. Additionally, you will find a link to the PDAC verification letter.
PDAC Verification Update: 1E95 Challenger Foot
Affecting: 1C50 Taleo, 1C60 Triton, 1C64 Triton HD & 1B1 Meridium
We are committed to providing timely updates on Ottobock feet and knees submitted to PDAC for coding verification. The following products have received coding verification effective November 13, 2020, and must be billed to Medicare with the stated HCPCS codes.
- Product: 1C50 Taleo
PDAC Verified Coding: L5981
Previous Codes: L5980+L5986
- Product: 1C60 Triton
PDAC Verified Coding: L5981
Previous Codes: L5980+L5986
- Product: 1C64 Triton HD
PDAC Verified Coding: L5981
Previous Codes: L5980+L5986
- Product: 1B1 Meridium
PDAC Verified Coding: L5973
Previous Codes: NO CHANGE IN CODING
PDAC Verified Products
For your convenience, we are maintaining a portal section that includes all Ottobock feet and microprocessor knees with PDAC verified coding. This document indicates the effective date, PDAC verified codes, and previously recommended coding for each product. Additionally, you will find a link to the PDAC verification letter.
Please remember, it is important for prosthetic providers to understand that once a decision is published by PDAC, that becomes the required coding for billing Medicare.
In preparation for the CMS requirement of prior authorization, additional coding guidelines were provided for the impacted lower limb HCPCS codes L5973, L5980, L5987, L5856, L5857, and L5858. As a result of the clarification provided in the new coding guidelines, the application of these codes to impacted prosthetic components is changing in many cases. Because many of these changes will result in a reduction in reimbursement, Ottobock is making pricing concessions to alleviate the potentially negative financial impact on your business.
We have made immediate pricing adjustments for 1C50 Taleo, 1C60 Triton, and 1C64 Triton HD. We believe the Taleo and Triton family of carbon fiber feet deliver the optimal outcomes your patients deserve. We are committed to you and we believe this pricing change will enable you to continue fitting Ottobock foot solutions. For detailed information regarding specific pricing changes, please contact your sales representative or log into your Ottobock online account.
We will continue to keep you informed regarding the verification status for all submitted applications. Additionally, please visit our online Reimbursement Portal for the most up-to-date information.
If you have any questions, please feel free to contact Ottobock Reimbursement Support.
Email us at reimbursement911@ottobock.com
Call us at 800 328 4058
Your Partners in O&P Care,
Ottobock Healthcare
PDAC Verification: Ottobock Knees and Feet
Effective January 1, 2021, Medicare has mandated that prosthetic products with the following lower limb prosthesis codes must have PDAC verification in order to bill Medicare:
- Microprocessor Knees: L5856, L5857, L5858
- Microprocessor Feet: L5973
- Flex Foot System: L5980
- Vertical Shock Feet: L5987
Ottobock products which currently have PDAC verified coding within these HCPCS codes are as follows. Please click on the product name to download a copy of the PDAC Coding Verification letter.
- 3C88/3C98 C-Leg Microprocessor Knee
- 1C61 Triton Vertical Shock
Additionally, Ottobock has submitted the following products for PDAC verification with the required codes:
- 3C60 Kenevo
- 1B1 Meridium
- 1C50 Taleo
- 1C51 Taleo Vertical Shock
- 1C52 Taleo Harmony
- 1C60 Triton
- 1C62 Triton Harmony
- 1C64 Triton HD
- 1E2 ProCarve Foot
- 1E58 Axtion DP
- 1E90 Sprinter
- 1E91 Runner
- 1E93 Runner Jr.
- 1E95 Challenger
It is important for prosthetic providers to understand that once a decision is finalized by PDAC, that becomes the required coding for billing Medicare, even before January 1, 2021.
Upon receipt of the code verification, Ottobock will email customers and publish a copy of the PDAC Coding Verification letter on our website. Prosthetic providers are responsible for proper coding of products, so we encourage you to visit our Reimbursement Portal frequently to determine if the Ottobock foot or microprocessor knee you are submitting for Medicare reimbursement has received PDAC-verified coding.
If you have any questions, please contact Ottobock Reimbursement Support. Email us at reimbursement911@ottobock.com, or call us at 800 328 4058. Visit our Reimbursement Portal online for the most up-to-date information available.
Your Partners in O&P Care,
Ottobock Healthcare
Logistics Update: Shipping has resumed from Louisville
A small number of logistics team members have been able to safely reopen our facility in Louisville this morning. Please place all critical orders requiring next day air shipping by 5:00pm CST. All orders unable to be shipped yesterday due to the closure will leave today with their original shipping method requested. If you need to change the shipping method of your order or if you have an urgent matter, please contact customer service. Thanks very much for your understanding and support.
-Your Partners in O&P Care
Ottobock Expands Taleo Foot Family with Launch of Taleo Vertical Shock and Taleo Harmony
AUSTIN, Texas (September 22, 2020) – We’re excited to introduce two new additions to our reputable Taleo carbon foot portfolio, Taleo Vertical Shock (VS) and Taleo Harmony. For active individuals who want relief for their residual limb, Taleo VS provides the clinician with more options for their patients. The unique functional ring unit effectively absorbs torsion (+/- 10°) to relieve the residual limb and enhance comfort in combination with vertical shock absorption (up to 15 mm).
Taleo Harmony extends the clinical toolbox by providing a foot option for individuals who want relief for their residual limb in addition to a firm hold and control over their prosthesis throughout the day. The integrated vacuum pump improves balance and reduces risk of falls1, provides better control over the prosthesis by reducing pistioning2, prevents skin irritation and delays in wound healing3, and reduces fluctuations in limb volume4.
Ottobock is committed to helping practitioners find the right solution for their patients. When it comes to choosing the right prosthetic foot for your patients, it’s more than a foot, it’s their foundation. Consider Ottobock as your full range foot provider.
Users are also excited by the new extended line. Daniela reports that with Taleo VS, she can “say goodbye… to knee pain.” For Flori, Taleo Harmony allows him to take “those small steps with turns” and turn them into steps that are “so soft and smooth.”
Learn more about Ottobock’s Taleo Family of Feet, and understand why with every fitting your goal is the same as ours: to craft the best possible solution for your patient.
For more information about our two new additions, visit our Taleo Foot Portfolio.
1 Samitier et al. 2014, Kahle et al. 2014, Kahle et al. 2013, Ferraro et al. 2011
2 Darter et al. 2016, Kahle et al. 2014, Kahle et al. 2013, Beil et al. 2002
3 Kahle et al. 2014, Hoskins et al. 2014, Traballesi et al. 2012, Brunelli et al. 2009
4 Kahle et al. 2014, Sanders et al. 2011, Goswami et al. 2003, Beil et al. 2002, Board et al. 2001
Update: Air & Ground Shipping Resume from Louisville Today
Update: After closing on 8/25/20, our supply and logistics center has reopened resumed normal operations.
Due to an organized protest and demonstration occurring within very close proximity of our supply & logistics center in Louisville, KY, we have decided to close the facility for today, August 25, 2020. Our first priority is the safety of our staff; therefore, no air or ground packages will be shipped on Tuesday, 8/25/2020.
We expect normal business operations to resume on Wednesday, August 26, 2020. If you have an urgent matter or need to change your shipping method, please contact our customer service team. Please accept our sincere apologies for any inconvenience this may cause. Thank you for your understanding regarding this matter.
-Your Partners in O&P Care
U.S. Marine Sees Life-Changing Impact with C-Brace
David Tupper - from U.S. Marine on the battlefield to fighting through his darkest days before learning about the revolutionary C-Brace® knee-ankle-foot orthosis.
David Tupper was doing what he always dreamed of doing; proudly serving his country in the U.S. Marines. He had always thought of going into the service and when 9-11 occurred, David knew this was his calling. He enlisted in the U.S. Marines and served four combat tours in Iraq and Afghanistan over nearly 11 years. David was on track for a successful career in the Marines having received several decorations for evacuating the wounded in Fallujah.
During his fourth and final tour in Afghanistan in 2012, he was severely injured in an IED attack. David was lifted five feet off the ground and thrown 13 feet causing a broken lower spine, shrapnel deep in his body and brain trauma. As a result of his injuries, David became paralyzed from the hip down and was unable to walk. Once a budding athlete and active husband and father, he was left to his own thoughts and darkest moments.
After seven years adapting to life in a wheelchair, David was introduced to the C-Brace orthosis by Ottobock. He hadn’t heard of the microprocessor-controlled technology but was immediately intrigued when his orthotist showed him a video of the product. After 45 minutes of ensuing the fit was perfect, David was out of his wheelchair and walking, unassisted, for the first time in years.
“Putting on the C-Brace for the first time was a feeling I could never relive again. Right then, it was me again” says David.
In this recorded live interview from the Ottobock Instagram account, David joined the "Ask Aaron" feed to discuss his road to recovery and the technology that allowed him to go from serving his country to serving his family.
Ottobock introduces Taleo Low Profile prosthetic foot
AUSTIN, Texas (April 13, 2020) – Ottobock HealthCare, a leading global supplier of high-quality medical devices, introduced Taleo Low Profile, the newest addition to its carbon foot portfolio. Taleo Low Profile is designed for active individuals (K3) with limited clearance who navigate varied indoor and outdoor environments and place a high value on effortless walking and the ability to go wherever life takes them.
Taleo Low Profile is the first addition to the highly successful Taleo line of feet. Ottobock's Research & Development team focused engineering efforts on designing a low-profile foot that will provide the same naturally smooth feeling experienced by individuals who wear Taleo.
"Taleo provides the flexibility and dynamic motion that people need in a low-profile prosthetic foot, without sacrificing functionality of the foot," said Mark Edwards, Ottobock Director of Professional and Clinical Services. "With consumers becoming increasingly knowledgeable about the different options in prosthetic feet, practitioners can gain greater confidence with the Taleo foot portfolio to fit a person with a foot that enables a smooth progression from heel strike to terminal stance."
For more information about the Taleo Low Profile, visit Taleo LP.
Salt Lake City Earthquake Impact on Ottobock Operations
Update: Salt Lake City operations back online
Thankfully no injuries were reported and every department is back up and running!
March 18, 2020
To our valued customers,
This morning our Salt Lake City operation was impacted by a 5.7 magnitude earthquake that caused damage to our building. Thankfully no injuries were reported, however, we were forced to temporarily shut down operations and send all employees home to make way for an assessment of building damage. Upon review, all services have been restored.
Our Salt Lake City operation includes After Sales Service, Returns Processing, Fabrication Services, Custom Liner Production and Carbon Foot Production. This disruption temporarily impacted our ability to support your needs in these areas, but please know we took immediate action to route all Fabrication and After Sales Service calls into our Austin-based Customer Service Team.
Please contact us should you have any questions.
Customer Service
US 800 328 4058 or USCustomerService@ottobock.com
Canada 800 665 3327 or CACustomerService@ottobock.com
*Please press 1 after the prompt for US and Canada Our phone lines and inboxes are monitored Monday-Friday 7:30 am – 6:00 PM CST.
Sincerely,
Brad Ruhl
Managing Director, Ottobock North America
First patient in North America fit with Ottobock’s FDA-cleared Myo Plus pattern recognition system
AUSTIN, Texas (November 11, 2019) – “Ottobock is excited to announce its newest advancement in upper-limb prosthetics, the Myo Plus pattern recognition system. Pattern recognition represents a new paradigm in the control of a myoelectric prosthesis. On October 29, 2019 Ottobock partnered with Hanger Clinic to fit the first person in North America with a transradial upper limb amputation with the newly FDA-cleared technology.
Traditionally, individuals with transradial upper limb amputation have been fit with myoelectric hands controlled by electromyographic (EMG) signals. The conventional transradial myoelectric prosthesis is designed with one or two electrodes that transmit the EMG signals produced by the flexor and extensor muscles in the residual limb. Some users have found these systems difficult to learn due to complex switching mechanisms required to change between the hand, wrist, or multiple grip patterns a hand may offer. In some cases, this difficulty may lead to poor adoption or even abandonment of the prosthesis.
Uniting artificial intelligence with the intuitive and innate EMG signals of the user, Myo Plus adapts to their natural movements versus requiring the user to adapt to the system. While standard myoelectric systems are limited to sensing only two muscle signals, Myo Plus employs up to 18 sensors, enabling multiple muscle signals to be captured as unique movement patterns, each individually assigned to control various functions of the prosthesis. For example, one pattern is used to open the hand, another to close it, a third pattern to rotate the wrist moving the palm of the hand up, and a fourth to rotate the wrist moving the palm of the hand down. Offering direct, convenient control of the prosthesis without any switching mechanisms required and the ability to fine-tune adjustments via an app, the Myo Plus system was designed with both the user and prosthetist in mind.
Chris Lake, L/CPO, FAAOP, an upper limb specialist with Hanger Clinic, fit the first North American patient, Mark Kirby, with the Myo Plus system in Hurst, Texas. Lake said, “Pattern recognition offers an alternative for patients who may not be a candidate for more traditional myoelectric prostheses, or do not possess the muscle capacity to operate the prosthesis in a meaningful way. To a certain extent, standard myoelectric systems require significant muscular acrobatics to fully operate a prosthesis. From an energy expenditure standpoint, pattern recognition means not burning as much physical or mental energy thus providing the patient with prosthetic function that is more intuitive and less taxing."
Kirby, who has used a standard two-site myoelectric prosthesis for more than ten years said, “I loved the graphic representation of what was possible. I instantly saw how I could make the prosthetic wrist rotate versus opening and closing the prosthetic hand. It took away the mystery. Since it's more accurate, I'm less frustrated because I've got less accidental movements. Before, I had to really concentrate on my finger muscles. Now I can feel them again. I'm feeling muscles I haven't felt in years."
The relationship between the clinician and patient was an important consideration in the design of the Myo Plus pattern recognition system. The patented Spider Plot feature, a graphic representation of muscle patterns, is one of the system’s most unique and valued features. Via the Myo Plus app, the patient and practitioner can watch live as the muscle patterns are displayed on a graph shaped like a spider web. This visual feedback was designed to make the programming process simple and interactive for both the patient and prosthetist.
"The visual nature of the Spider Plot feature in Myo Plus makes it intuitive for Mark to learn how to use the technology, as well as understand the very finite changes he’s making as he contracts his muscles. It provides an instant connection between his muscles and his phantom hand,” said Lake.
If you would like additional information on the Myo Plus pattern recognition system, please contact Ottobock North America at 800 328 4058.
About Ottobock
For 100 years Ottobock, the German global technology leader, has been developing med-tech products and fitting solutions for people with limited mobility in the areas of Prosthetics, Orthotics and Human Mobility. The company’s international activities are coordinated from the head office in Duderstadt/Germany. Ottobock has been investing, employing, researching and developing in the US since 1958 for the benefit of people with impaired mobility. Subsidiaries in 58 countries offer “Made by Ottobock” quality worldwide and employ more than 7,000 people.
Media Contacts
Cali Solorio│Ottobock North America│ 512 806 2655 │ Cali.Solorio@ottobock.com
Ottobock introduces the Agilium® Vantage
AUSTIN, Texas (October 18, 2019) – “Ottobock HealthCare, a leading global supplier of high-quality medical devices, introduced the latest addition to its orthotics portfolio: The Agilium® Vantage. The Agilium Vantage is a low-profile, wraparound knee brace designed to provide lasting pain relief for patients with mild unicompartmental osteoarthritis (OA).
Building on the Agilium line of OA knee braces, the Agilium Vantage features a lightweight design for everyday use, recreational activities, and sports. Patients can don and doff the brace easily using the four adjustable, numbered straps. The dynamic Y-force strap system unloads the knee with a single pull, while reducing pain and providing stability. An anti-rotation strap keeps the brace in place, and the breathable material prevents popliteal bunching for increased patient compliance and comfort.
The Agilium Vantage is now available for order in three trimmable sizes, allowing practitioners to reduce inventory and save on costs.
“A brace is a key component in the treatment of osteoarthritis-related knee pain,” says Gloria So, Market Manager of Activity and Sports Medicine at Ottobock. “Osteoarthritis is one of the most common joint disorders worldwide, and the Agilium Vantage is designed to improve functional outcomes for patients so they can resume their daily activities with confidence.”
For more information about the Agilium Vantage, visit https://www.ottobockus.com/orthotics/solution-overview/agiliumvantagephysician/.
2019 Thranhardt Award Winners Announced
WASHINGTON, D.C. (July 17, 2019) – “The American Orthotic and Prosthetic Association is proud to announce the 2019 Thranhardt Award winners. They represent the best in orthotic and prosthetic patient care,” said Jim Weber, MBA, President, American Orthotic and Prosthetic Association. Among this year's winners is Dr. Andreas Hahn, PhD, MSc, who is the corporate vice president of clinical research and services at Ottobock.
“The Thranhardt Lectures have become a highpoint for National Assembly attendees. We look forward to celebrating the winners in San Diego," Weber said.
The two other recipients are Tiffany Graham, MSPO, CPO/L, FAAOP and Kelly Millay, MPO. Graham and Millay will present "Significant Factors in Orthotic Treatment of Asymmetrical Brachycephaly." Graham has focused her career in pediatric orthotics and has over 10 years of experience with treating cranial deformations. She is an instructor at the University of Texas Southwestern Medical Center, Vice-Chair of the Academy’s Craniofacial Society, and recipient of the 2018 Thranhardt Award. Millay is currently an orthotic resident at University of California at San Francisco and a member of the Academy's Craniofacial Society. Her research interest focuses in cranial deformations.
Hahn will present The Use of Health Economic Instruments to Evaluate Prosthetic Services: Experience with Over 400 Patients in Germany and India. He joined Ottobock in 2004 and currently holds the global corporate responsibility for Ottobock’s Clinical Research activities. Hahn is a physicist at Oxford University, has over 20 years professional experience in leading positions in the Medical Device Industry, and was a 2014 Thranhardt Award winner.
The new 1C50 Taleo® prosthetic foot
AUSTIN, Texas (May 27, 2019) – For 100 years, Ottobock has been at the forefront of prosthetic advancement. Today, Ottobock announces the launch of a new prosthetic foot, Taleo, that continues this tradition. It was developed based on direct feedback from clinicians and end users to address the specific needs of active individuals and is the newest member of the Ottobock carbon foot portfolio, joining the Trias® and Triton® foot family.
The Taleo foot is designed for those amputees who lead an active life either at home or in the great outdoors and place a high value on effortless walking and freedom to go everywhere. With its flexible, dual carbon springs, the Taleo allows for both a smooth rollover and dynamic gait pattern. A springy and efficient return of energy at toe-off comes from its progressive spring characteristics. Therefore, users can effortlessly cover longer distances while changing walking speeds as needed throughout the entire day. Taleo comfortably adapts to the user’s gait characteristics. The flexible connection of the springs at the forefoot ensure stable and comfortable walking on uneven or hilly terrain. Due to the broad range of heel wedges, the clinician can improve the comfort at heel strike by individually tailoring the shock absorbing characteristics of the prosthetic foot. Even walking barefoot for short periods is much more pleasant. As the Taleo is protected against fresh, salt and chlorine water users can enjoy their hobbies without thinking twice.
In addition to a unique spring design, the Taleo also features other important design elements for both the clinician and the user such as:
- Water runoff channels on the pyramid and drain holes in the foot shell make it easy to clean after being in the water
- Customizable heel dampening with 3 different stiffness options
- Alignment marks on the foot shell simplify bench alignment procedure
- Slim design of the pyramid adapter makes it easier to create an attractive cosmetic cover The Taleo is suitable for users weighing up to 330 lbs (150 kg) and is available in sizes from 22 to 30 cm.
About Ottobock
Ottobock uses innovative technology, superior service, and world-class education to help people with physical mobility challenges. Established 100 years ago in Germany, Ottobock opened its doors in the U.S. in 1958 and in Canada in 1978. Currently in its third generation as a privately held company, Ottobock offers products and services to help people maintain or regain their freedom of movement.
Ottobock Issues Statement on U.S. Federal Trade Commission Decision Regarding Acquisition of Freedom Innovations
AUSTIN, Texas (May 7, 2019) – Today, the U.S. Federal Trade Commission released the FTC’s Administrative Law Judge’s Initial Decision regarding the acquisition of Freedom Innovations by Ottobock, recommending to the FTC Commission the divestiture of certain assets of Freedom Innovations. Ottobock acquired Freedom Innovations in September of 2017 with the goal of strengthening the prosthetic product choices and technology for the benefit of amputees and consumers. Ottobock expresses its disappointment in the Initial Decision. Ottobock will continue to work in a collaborative manner with the FTC to promptly reach a mutually beneficial resolution.
Ottobock is considering all options to protect our ability to deliver greater consumer choice, improve product quality and fuel innovation. As Ottobock evaluates potential actions, Freedom Innovations will continue to operate as an independent business. “Ottobock is committed to serving customers by providing them with a selection of the most innovative products to meet their needs. As we have throughout our 100-year history, we will persist in exploring and advancing all efforts necessary to ensure patients have access to the smart technology and care that they deserve, and will actively pursue strategies that deliver on this mission,” says Brad Ruhl, Managing Director of Otto Bock HealthCare North America.
About Ottobock
For 100 years Ottobock, the German global technology leader, has been developing med-tech products and fitting solutions for people with limited mobility in the areas of Prosthetics, Orthotics and Human Mobility and provides patient care in its MedicalCare division. In 2018 the company transferred its biomechanical expertise to applications for industrial use with its exoskeleton Paexo. The company’s international activities are coordinated from the head office in Duderstadt/Germany. Ottobock has been investing, employing, researching and developing in the US since 1958 for the benefit of people with impaired mobility and has since then always been a good corporate citizen. Subsidiaries in 58 countries offer “Made by Ottobock” quality worldwide and employ more than 7,000 people. Ottobock has supported the Paralympic Games with its technical expertise since 1988.
Media Contact
Albert Li
General Counsel
Ottobock
11501 Alterra Parkway, Suite 600
Austin, Texas 78758
Phone: 800 328 4058 ext. 2660
Email: Albert.Li@ottobock.com
Ottobock develops new Skeo Sealing Liner for above-knee amputees
AUSTIN, Texas (May 1, 2019) – Ottobock, a global industry leading supplier and manufacturer of innovative and technology-driven prosthetic solutions, has launched the new 6Y110 Skeo Sealing Liner. Designed for use with one-way valves or vacuum prosthetic suspension systems, the Skeo Sealing Liner allows individuals with above-knee and knee-disarticulation amputations to don their prostheses easily without an aid.
The incredible ease with which the Skeo Sealing can be put on and taken off is due to its smooth exterior surface. Because the liner is made without a fabric cover, the liner can easily be cut to size, is easy to clean, and dries quickly. The textured inner surface of the liner incorporates Skinguard® antibacterial additive to prevent the growth of odor-causing bacteria and feels comfortable and secure against the skin without sticking.
The Skeo Sealing Liner features a single, sturdy silicone ring that reliably maintains vacuum in the socket, and a no-stretch matrix integrated along the length of the liner minimizes pistoning inside the socket to ensure excellent tissue management.
The Skeo Sealing Liner is optimized for comfort, security, ease of use, and independence.
About Ottobock: Ottobock uses innovative technology, superior service, and world-class education to help people with physical mobility challenges. Established 100 years ago in Germany, Ottobock opened its doors in the U.S. in 1958 and in Canada in 1978. Currently in its third generation as a privately held company, Ottobock offers a range of products and services to help people maintain or regain their freedom of movement.
Paralympic athletes and fashion experts will speak at Ottobock’s two SXSW 2019 panels
AUSTIN, TEXAS, USA (FEBRUARY 22, 2019) – Ottobock, a global industry leader in prosthetics, will host two panel sessions at the annual South by Southwest® (SXSW®) festival in Austin, Texas on March 8, 2019: “Adaptive Athletics: The Rise of the Super Athlete?” and “How Adaptive Design is Transforming Brands.” Panelists for the two sessions range from Paralympic athletes to adaptive fashion models and influencers. Additionally, qualified media are invited to attend an exclusive press event on March 7, 2019 at Ottobock’s North American headquarters in Austin, Texas – media will have early access to speak with panelists and ask questions the day before SXSW begins.
Ottobock’s first SXSW 2019 panel, “How Adaptive Design is Transforming Brands,” will take place on March 8 from 11:00 a.m. to 12:00 p.m. at the Four Seasons Ballroom AB (98 San Jacinto Blvd.). The session will discuss how fashion’s biggest brands are working with designers to create mainstream, adaptive clothing that addresses the needs and challenges of people with disabilities and chronic illnesses. Panelists for this session include: Cacsmy Brutus, model and spokesperson at JAG Models Molly Kettle, director at Zappos Adaptive; Melissa Langley, marketing communications strategist at Ottobock; and Mindy Scheier, founder of Runway of Dreams.
Ottobock’s second SXSW 2019 panel, “Adaptive Athletics: The Rise of the Super Athlete?,” will take place on March 8 from 5:00 p.m. to 6:00 p.m. at the JW Marriott Salon 1-2 (110 East 2nd Street). The session will discuss the controversy of whether technology provides an unfair advantage or disadvantage to athletes with disabilities. Panelists for this session include: Scout Bassett, Paralympian and motivational speaker; Brian Frasure, CP, senior clinical specialist at Ottobock and former Paralympian; Alena Grabowski, Ph.D., assistant professor at University of Colorado Boulder; and Blake Leeper, Paralympic track and field athlete.
“Ottobock is excited to participate in SXSW for the fourth year in a row,” said Scott Schneider, Vice President of Government, Medical Affairs and Future Development at Ottobock “For 100 years, Ottobock has delivered innovative medical technology products and services to help people maintain and regain their freedom of movement. Our goal is to continue to maximize independence for life for people with mobility challenges, and further support the advancement of prosthetics and orthotics.”
SXSW 2019 will take place March 8-17, 2019. For more information, please visit www.sxsw.com. To register for the event, please visit www.sxsw.com/attend. Ottobock offers a complete line of orthotic and prosthetic solutions.